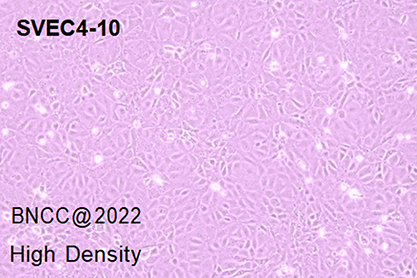
SVEC4-10
mouse lymph node vascular epithelioid cells
BNCC number: 357598
Growth properties : Adherent growth
Growth conditions : 37 ℃,5% CO2
Complete medium : 90% DMEM-H + 10% FBS
Cryopreservation conditions: 50% DMEM-H + 40% FBS + 10% DMSO
Receiving Notices:if any abnormality is found on the day of receiving goods, please contact customer service within 24 hours. overdue goods will be deemed to be good! Freezing storage tube form: store in a refrigerator at -80 ℃ in time after receiving the goods. If it is not used for a long time, it should be transferred to liquid nitrogen overnight. During resuscitation, each tube shall not be retained once used up.T25 form: after receiving the goods, let it stand in the incubator for 2-3 hours, and then perform routine operation on the cells. Please strictly follow this instruction, otherwise no reissue service will be provided if cell inactivation is caused.
Recovery steps:
① take out the frozen storage tube from liquid nitrogen or -80 ℃ and put it into PE gloves, quickly subtract into a 37 ℃ water bath pot, shake the frozen storage tube to accelerate dissolution, and it is advisable to dissolve it completely within 1 minute.
② add the dissolved cell fluid into a centrifuge tube filled with 9ml of complete culture medium in an ultra-clean table, centrifuge at 1000-1200rpm/min for 5 minutes, discard the supernatant, and resuspend the cells with 1-2ml of complete culture medium.
③ then add the cell suspension to T25 bottle containing 5-6mL of complete medium and put it into an incubator for culture.
Cell subculture:
① absorb the old culture solution, clean PBS twice, and add 1-2mL pancreatin (0.25% Trypsin + 0.02% EDTA);
② observe the digestion under the microscope. when the cell edge shrinks and the wall is loose (a straw can be used to suck up some pancreatin and gently blow somewhere in the cell layer, and the cell layer can be seen to fall off with naked eyes, I .e. digestion is completed, otherwise digestion is continued), directly suck off pancreatin, add 5-6ml of complete culture medium, gently blow the cell layer, blow off the cell layer and blow it off.
③ divide the cell suspension into a new T25 bottle according to a ratio of 1:2, add appropriate complete culture medium, beat the cell suspension evenly, and culture in an incubator.
④ pay attention to the change of pH value and cell density of the culture medium, change the liquid regularly (2-3 times a week), and repeat the passage operation or cryopreservation when the cell density reaches 80%-90%.
Notes:
①The cells are density-dependent, and are passaged for the first time (density reaches 80%). It is recommended to pass 1:2 (maintain cell density), and preferentially in the T25 bottle.
② if the culture bottle is sealed, put it into an incubator for cultivation after treatment, and remember to loosen the cap of the culture bottle.
Recovery record: According to the recovery instructions, the results of the cell recovery are reported as follows:
| Item |
quality standard |
recovery record |
| viability: |
adherence is observed in 18 hours, the cell adherence rate ≥ 80.0% in 68hours |
adherence is observed in 18 hours, the cell adherence rate ≥ 80.0% in 72hours |
| cell morphology: |
epithelial cell-like |
epithelial cell-like, polygonal, fusiform |
| attached figure: |
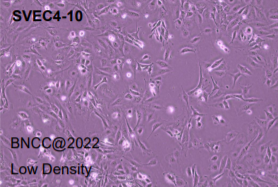 |
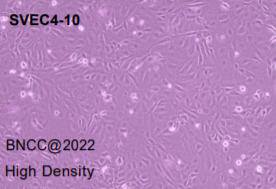 |
| Conclusion: |
good viability, and no abnormal cell morphology, qualified |

 info@bncc.com
info@bncc.com
 - English
- English
 - Japanese
- Japanese