1.Description
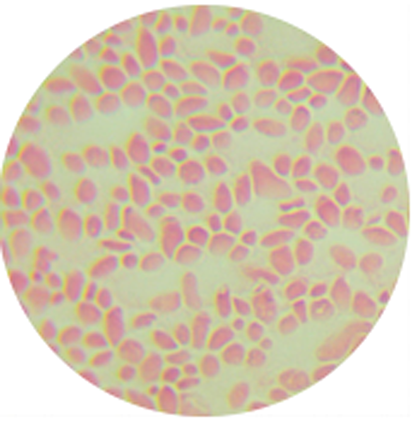
1. Name:Vibrio harveyi
2. BNCC No.:336937
3. Biosafety level : 4
2. Storage conditions:
Storage of freezed dried ampoule and agar slant at 2°C to 8°C
3.Growth Conditions
1, TSA + 5% defibrinated sheep blood: tryptone (casein trypsin digest) 15.0g, soybean peptone (soybean powder papain digest) 5.0g, sodium chloride 5.0g, agar 15.0g,pH7.3±0.2. 121 ℃,15min. After sterilization, 5% defiber sheep blood was added under aseptic conditions. Or sea water agar 2216.
2.Atmosphere:aerobic
3. Temperature: 30 ℃
4.Notes:
1.Normal culturing time, 1-2days for bacterial, 3 days for yeast, 5-7days for mould, 7-10days for fungal.
2.Agar slant shall be inoculated asap, and do not keep the storage for more than 3 months.
3.Please recover the strains in strict accordance with this instruction, otherwise the replacement of the strain are not be available in case of viability loss caused by different media or growth conditions.
4.Waste generated from the handling process should be discarded after high-pressure sterilization.

 info@bncc.com
info@bncc.com
 - English
- English
 - Japanese
- Japanese